Research
The digestive tract provides an important interface in the communication between environmental factors, the immune system and the host metabolism. The incidence and prevalence of chronic inflammatory and metabolic diseases is increasing dramatically in industrialized countries.
The gut epithelium is a multicellular interface located in close proximity to a complex and dense microbial milieu. Simultaneously intestinal epithelial cells absorb nutrients, and form a physical and immune-mediated barrier against adverse components of the luminal environment. The intestinal epithelium represents the most regenerative tissue in the human body and the interaction of microbes and their metabolites with the intestinal epithelium has numerous effects on body functions and the development of inflammatory and metabolic diseases.
The intestinal microbiome is defined as the totality of all microorganisms (microbiota), including bacteria, archaea, viruses, yeasts and protozoa, and their physio-chemical properties in the intestine. The metagenome describes the entirety of all microbial genomes and provides an insight into the functional repertoire of the microbiota. The intestinal microbiome varies greatly between individuals and is influenced throughout life by many environmental factors such as diet, environment, health status and drug intake. The gut microbiome is characterized by a high degree of stability (resilience) in healthy individuals, while adverse compositional and functional changes (dysbiosis) in the microbiome are causally involved in the pathogenesis of chronic inflammatory bowel diseases (IBD), such as Crohn's disease and ulcerative colitis, and colorectal cancer (CRC). Diet is an environmental factor that influences the composition and activity of the gut microbiota and thus exerts a direct or indirect influence on the onset and course of diseases in the digestive tract. Investigations into the interaction between nutrition and the microbiome are one of the key focus areas at the Chair of Nutrition and Immunology.
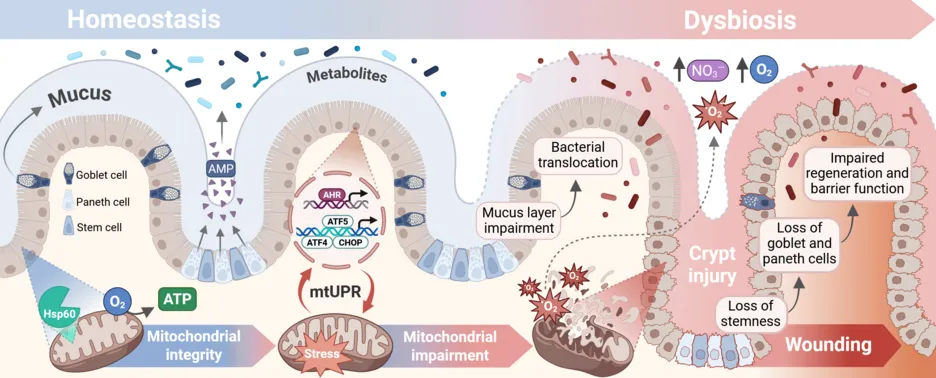
The gut epithelium represents the most regenerative tissue in the human body, located in proximity to the dense and functionally diverse microbial milieu. Maintaining the immunological and physical barrier function of the gut epithelium is therefore of immense importance for the host. Chronic stress signaling and mitochondrial dysfunction in intestinal epithelial cells are emerging concepts in the pathogenesis of inflammation and tumorigenesis. Metabolic reprogramming of the epithelium is also implicated in shaping an adverse microbial milieu, supporting the hypothesis that microbial-metabolic circuits have a decisive influence on the regenerative capacity of the intestinal tissue and the development or recurrence of intestinal diseases. Investigations to the interplay between the microbiome and the intestinal epithelium in shaping health and diseases are another important focus of the scientific work at the Chair of Nutrition and Immunology.
Inflammatory bowel diseases (IBD), including Crohn's disease and ulcerative colitis, are multifactorial inflammatory diseases that are driven by a combination of genetic predisposition and environmental factors. Ulcerative colitis exclusively affects the large intestine, whose mucosa is continuously inflamed from the rectum towards the proximal colon. The discontinuous and segmental appearance of Crohn's disease affects the entire gastrointestinal tract and all layers of the intestine. Symptoms usually occur in flares followed by periods of remission and range from clinically symptom-free remission, which may nevertheless be accompanied by persistent subclinical inflammation, to clinical symptoms with abdominal pain and diarrhea, bloody stools, intestinal obstruction and fistula formation, and even the development of cancer. IBD patients experience an uncontrolled and excessive immune response to their own intestinal microbiota, accompanied by impaired barrier and innate immune functions. Reasons include defects in the regulation of cellular stress and mitochondrial function, which can lead to abnormal differentiation and regeneration of intestinal epithelium. Incompletely healed lesions in the intestine lead to chronic inflammation and tissue remodeling, and even the development of cancer. The microbiome plays a decisive role in the pathogenesis of IBD and CRC. Prof. Haller and his team are involved in clinical trials and develop novel animal to better understand the detrimental and protective influence of commensal microbes and nutritional factors in modulating chronic inflammation and tumor development in the digestive tract.
Prof. Haller and his team are involved in prospective cohorts and conduct controlled intervention studies in newborns, healthy volunteers and patients. The aim of these human studies is to better understand the influence of nutrition or specific food components and other environmental factors on the composition and function of the gut microbiome.
Ongoing projects:
Circadian regulation of the gut microbiome across the lifespan
Influence of pro- and prebiotics on the microbiome in the infant gut
Therapeutic impact of exclusive enteral nutrition (EEN) in pediatric Crohn's disease
Influence of refined and processed foods on the gut microbiome
Ongoing intervention studies:
Influence of the microalga Chlorella sorokiniana on microbiome-host interactions in humans (ZIEL PhD program and TUMCreate Proteins4Singapore)
Effect of dietary fiber and polyphenols from sweet lupine and sunflower flour on the microbiome (FEI)
Fecal microbiota transfer for maintenance of remission after induction treatment with exclusive enteral nutrition in Crohn's disease (EN-RICH, The Leona M. and Harry B. Helmsley Charitable Trust)
Selected publications:
Heppner N, Reitmeier S, Heddes M, Vig Merino M, Schwartz L, Dietrich A, List M, Gigl M, Meng C, R van der Veen D, Schirmer M, Kleigrewe K, Omer H, Kiessling S, Haller D*. Diurnal rhythmicity of infant fecal microbiota and metabolite profiles: a randomized controlled interventional trial with infant formula. Cell Host & Microbe. 2024 April 10.
Reitmeier S, Kiessling S, Clavel T, List M, Almeida EL, Ghosh TS, Neuhaus K, Grallert H, Linseisen J, Skurk T, Brandl B, Breuninger TA, Troll M, Rathmann W, Linkohr B, Hauner H, Laudes M, Franke A, Le Roy CI, Bell JT, Spector T, Baumbach J, O'Toole PW, Peters A, Haller D*. Arrhythmic Gut Microbiome Signatures Predict Risk of Type 2 Diabetes. Cell Host & Microbe. 2020 Jun 29:1931-3128(20)30343-7.
Lee T, Clavel T, Smirnov K, Schmidt A, Lagkouvardos I, Walker A, Lucio M, Michalke B, Schmitt-Kopplin P, Fedorak R, Haller D*. Oral versus intravenous iron replacement therapy distinctly alters the gut microbiota and metabolome in patients with IBD. Gut. 2017;66:863-871.
Bazanella M, Maier TV, Clavel T, Lagkouvardos I, Lucio M, Maldonado-Gòmez MX, Autran C, Walter J, Bode L, Schmitt-Kopplin P, Haller D*. Randomized controlled trial on the impact of early-life intervention with bifidobacteria on the healthy infant fecal microbiota and metabolome. Am J Clin Nutr. 2017 Nov;106(5):1274-1286.
Rath E, Berger E, Messlik A, Nunes T, Liu B, Kim SC, Hoogenraad N, Sans M, Sartor RB, Haller D*. Induction of dsRNA-activated protein kinase links mitochondrial unfolded protein response to the pathogenesis of intestinal inflammation. Gut 2012;61(9):1269-78
Prof. Haller and his team develop new animal models for chronic intestinal diseases. Investigations focus on the role of microbe-host interactions in the pathogenesis of chronic inflammatory diseases, such as Crohn's disease and ulcerative colitis, and colorectal cancer. Developing of and working with germ-free animal models for inflammation and cancer are a hallmark of the research group. The selective colonization of these animal models with the stool microbiome of patients and synthetic microbial consortia enable us to functionally and mechanistically characterize microbe-host interactions.
Ongoing projects:
Importance of mitochondria in the intestinal epithelium on wound healing and inflammation
Activating Transcription Factor (ATF)-6 in the development of colon cancer
Influence of processed foods on the microbiome, inflammation and cancer
Nutritional therapy through exclusive enteral nutrition (EEN)
Animal models:
Organ-specific/germ-free Hsp60-/- (intestine, liver, fat) inflammation models
Organ-specific/germ-free nAtf6 transgenes (intestine, liver) Cancer models
Crohn's disease inflammation models in mice and pigs (TNFDARE)
Selected publications:
Urbauer E, Aguanno D, Mindermann N, Omer H, Metwaly A, Krammel T, Faro T, Remke M, Reitmeier S, Bärthel S, Kersting J, Huang Z, Xian F, Schmidt M, Saur D, Huber S, Stecher B, List M, Gómez-Varela D, Steiger K, Allez M, Rath E, Haller D*. Mitochondrial perturbation in the intestine causes microbiota-dependent injury and gene signatures discriminative of inflammatory disease. Cell Host & Microbe 2024 Jul 10 doi: 10.1016/j.chom.2024.06.013
Coleman OI, Sorbie A, Bierwirth S, Kövilein J, von Stern M, Köhler N, Wirbel J, Schmidt C, Kacprowski T, Dunkel A, Pauling JK, Plagge J, Mediel-Cuadra D, Wagner S, Peng T, Metzler T, Schafmayer C, Hinz S, Röder C, Röcken C, Stecher B, Rosenstiel P, Steiger K, Jesinghaus M, Liebisch G, Ecker J, Zeller G, Janssen KP, Haller D*. ATF6 activation alters colonic lipid metabolism causing tumor-associated microbial adaptation. 10.1101/2023.11.03.565267 bioRxiv
Winogrodzki T, Metwaly A, Grodziecki A, Liang W, Klinger B, Flisikowska T, Fischer K, Flisikowski K, Steiger K, Haller D*, Schnieke A*. TFF DARE pigs: A translational Crohn’s diease model. J Crohns Colitis. 2023 Jul 5;17(7):1128-1138.
Coleman OI, Lobner EM, Bierwirth S, Sorbie A, Waldschmitt N, Rath E, Berger E, Lagkouvardos I, Clavel T, McCoy KD, Weber A, Heikenwalder M, Janssen KP, Haller D*. Activated ATF6 Induces Intestinal Dysbiosis and Innate Immune Response to Promote Colorectal Tumorigenesis. Gastroenterology. 2018 Nov;155(5):1539-1552.
Yuan D, Huang S, Berger E, (…), Haller D*, Heikenwalder M*. Kupffer Cell-Derived Tnf Triggers Cholangiocellular Tumorigenesis through JNK due to Chronic Mitochondrial Dysfunction and ROS. Cancer Cell. 2017;31:771-789.
Schaubeck M, Clavel T, Calasan J, Lagkouvardos I, Haange SB, Jehmlich N, Basic M, Dupont A, Hornef M, von Bergen M, Bleich A, Haller D*. Dysbiotic gut microbiota causes transmissible Crohn's disease-like ileitis independent of failure in antimicrobial defence. Gut. 2016;65:225-37.
Waldschmitt N, Berger E, Rath E, Sartor RB, Weigmann B, Heikenwalder M, Gerhard M, Janssen KP, Haller D*. C/EBP homologous protein inhibits tissue repair in response to gut injury and is inversely regulated with chronic inflammation. Mucosal Immunol. 2014 Nov;7(6):1452-66.
The intestinal epithelium forms an essential barrier and acts as an interactive interface between the microbiome and nutrition. Cell organelles such as the endoplasmic reticulum (ER) and mitochondria play an important role in the ability of the intestinal epithelium to regenerate. A central working hypothesis of the chair is that signaling factors (e.g. Activating Transcription Factor 6) of the ER-associated unfolded protein response (ER-UPR) and stress mechanisms in mitochondria control the ability of intestinal epithelial cells to differentiate and regenerate. New mouse models and cell models are used to develop new approaches for the prevention and therapy of inflammatory diseases and cancer in the intestine.
Ongoing projects:
Repurposing of drugs to improve wound healing in the intestine
Effect of H2S (Desulfovibrio ssp.) on tumorigenesis in the intestine
Identification of protective metabolites from the intestinal microbiome
Effect of fatty acids on tumorigenesis
Methods and model systems:
Intestinal organoids from mouse and pig models
Continuous culture systems for the stool microbiome (chemostat)
16S and metagenome sequencing of stool and biopsy samples
Fluorescence-based analysis of microbes and tissue
Selected publications:
Häcker D, Siebert K, Smith BJ, Köhler N, Heimes H, Metwaly A, Mahapatra A, Hölz H; De Zen F, Heetmeyer J, Socas K, Le Thi G, Meng C, Kleigrewe K, Pauling JK, Neuhaus K, List M, Pollard K, Schwerd T*, Haller D*. Exclusive Enteral Nutrition Initiates Protective Microbiome Changes to Induce Remission in Pediatric Crohn's Disease. 10.1101/2023.12.21.23300351 medRxiv
Metwaly A, Jovic J, Waldschmitt N, Khaloian S, Heimes H, Häcker D, Ahmed M, Hammoudi N, Le Bourhis L, Mayorgas A, Siebert K, Basic M, Schwerd T, Allez M, Panes J, Salas A, Bleich A, Zeissig S, Schnupf P, Cominelli F, Haller D*. Diet prevents the expanson of segmented filamentous bacteria and ileo-colonic inflammation in a model of Crohn’s disease. Microbiome 2023 Mar 31;11(1):66.
Khaloian S, Rath E, Hammoudi N, Gleisinger E, Blutke A, Giesbertz P, Berger E, Metwaly A, Waldschmitt N, Allez M, Haller D*. Mitochondrial impairment drives intestinal stem cell transition into dysfunctional Paneth cells predicting Crohn's disease recurrence. Gut. 2020 Nov;69(11):1939-1951.
Berger E, Rath E, Yuan D, Waldschmitt N, Khaloian S, Allgauer M, Staszewski O, Lobner EM, Schottl T, Giesbertz P, Coleman OI, Prinz M, Weber A, Gerhard M, Klingenspor M, Janssen KP, Heikenwalder M, Haller D*. Mitochondrial function controls intestinal epithelial stemness and proliferation. Nature Commun. 2016;7:13171.
von Schillde MA, Hormannsperger G, Weiher M, Alpert CA, Hahne H, Bauerl C, van Huynegem K, Steidler L, Hrncir T, Perez-Martinez G, Kuster B, Haller D*. Lactocepin secreted by Lactobacillus exerts anti-inflammatory effects by selectively degrading proinflammatory chemokines. Cell Host & Microbe. 2012;11:387-96.
Ocvirk S, Sava IG, Steck N, Roh JH, Tchaptchet S, Bao Y, Hansen JJ, Huebner J, Murray BE, Sartor RB, Haller D*. Surface-associated lipoproteins link Enterococcus faecalis virulence to colitogenic activity in IL-10-deficient mice independent of their expression levels. PLoS Pathog.2015 Jun 12;11(6):e1004911
The following reviews introduce the research visions of the Chair of Nutrition and Immunology:
Metwaly A, Reitmeier S, Haller D*. Microbiome risk profiles as disease biomarkers for inflammatory and metabolic disorders. Nat Rev Gastroenterol Hepatol. 2022;19(6):383-397.
Rath E, Moschetta A, Haller D*. Mitochondrial function – gatekeeper of intestinal epithelial cell homeostasis. Nat Rev Gastroenterol Hepatol. 2018 Aug;15(8):497-516.
Renz H*, von Mutius E, Brandtzaeg P, Cookson WO, Autenrieth IB, Haller D*. Gene-environment interaction in chronic inflammatory disease. Nat Immunol. 2011 Apr;12(4):273-7.