
BIOACTIVES
From natural product chemistry to biological function
Discovery of natural products with diverse, but specific bioactivities such as, e.g. antioxidative, antidiarrheal, antiviral, anti-diabetic, and anticancer compounds, mycotoxins, and bacterial toxins in food products and plant materials is performed by means of bioassay-guided fractionation using cellular tests and/or chemical assays in combination with instrumental analysis (LC-TOF-MS, 1D-/2D-NMR, CD etc.). Thereafter, quantitation of bioactives is executed via LC-MS/MS or qNMR and their metabolic fate is studied in intervention studies.
Example 1: Chemodiversity of cereulide, the emetic toxin of Bacillus cereus
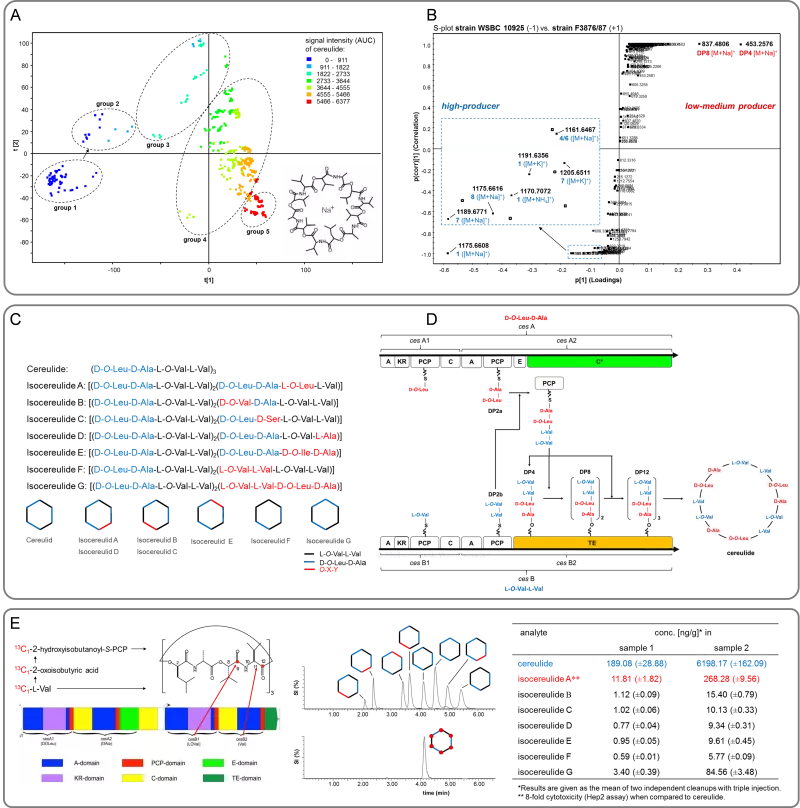
Food-borne intoxications are increasingly caused by the dodecadepsipeptide cereulide (L-O-Val-L-Val-D-O-Leu-D-Ala)3, the emetic toxin produced by Bacillus cereus (Fig. 1A/C). As such intoxications pose a health risk to humans, a comprehensive understanding on the chemodiversity of this toxin is mandatory for the reliable risk assessment of B. cereus toxins in foods. A fast and robust high-throughput UPLC-ToF-MS profiling method was developed and successfully applied to discriminate a total of 78 Bacillus cereus strains into no (group 1, Fig. 1A), low/medium (group 2/3) and high (group 4/5) producers of the emetic toxin cereulide.
Mass spectrometric screening and multivariate analysis (Fig. 1B) showed a series of at least 18 cereulide variants, among which the previously unknown isocereulides A-G (Fig. 1C) were determined for the first time by means of UPLC-ToF-MS and ion-trap MSn sequencing, 13C-labelling experiments, and post-hydrolytic dipeptide and enantioselective amino acid analysis. Most intriguingly, the isocereulides were found to differ widely in their cell toxicity, correlating with their ionophoric properties; e.g. purified isocereulide A showed about 8-fold higher cytotoxicity than purified cereulide in the HEp-2 assay and induced an immediate breakdown of bilayer membranes.
In comparison to the high producers (group 4/5, Fig. 1A), the UPLC-ToF-MS analysis of low/medium producers (group 2/3) revealed tetra-, octa-, and dodecapeptides of a different sequence, namely (L-O-Val-L-Val-D-O-Leu-D-Ala)1-3, as intermediates of cereulide biosynthesis (Fig. 1B). Surprisingly, also di-, hexa-, and decadepsipeptides were identified, which, together with the structures of the previously reported isocereulides E, F, and G, do not correlate with the currently proposed mechanism for cereulide biosynthesis and violate the canonical NRPS biosynthetic logic. UPLC-ToF-MS metabolite analysis and bioinformatic gene cluster analysis highlighted dipeptides rather than single amino or hydroxy acids as the basic modules in tetradepsipeptide assembly and proposed the CesA C-terminal C* domain and the CesB C-terminal TE domain to function as a cooperative esterification and depsipeptide elongation center repeatedly recruiting the action of the C* domain to oligomerize tetradepsipeptides prior to the release of cereulide from the TE domain by macrocyclization (Fig. 1D).
For the first time, cereulide as well as its 13C6-isotopologue was prepared by means of a biosynthetic approach using a B. cereus culture, followed by a rapid but efficient down-stream purification. Finally, a rapid, sensitive, and robust stable isotope dilution assay (SIDA) was developed for the combined quantitation of isocereulides and cereulide by means of UPLC-MS/MS (Fig. 1E). The developed method served as basis for the European iso standard ISO 18465:2017: "Microbiology of the food chain - Quantitative determination of emetic toxin (Cereulide) using LC-MS". Application of this method to suspicious noodle samples which caused intoxications to children revealed elevated levels of (iso)cereulide(s). In consequence, this SIDA method might add an important contribution to the knowledge-based risk assessment of B. cereus toxins in foods.
Example 2: Natural plant inhibitors of the sodium-dependent glucose transporter (SGLT1)
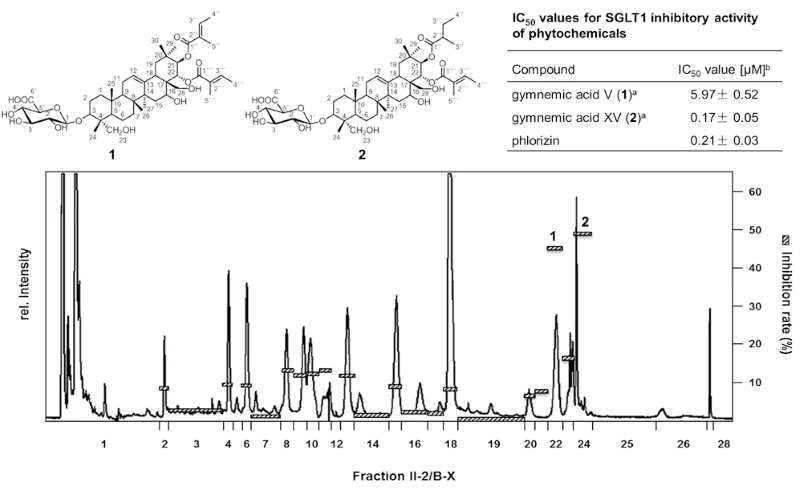
As Traditional Chinese Medicine is using distinct plant materials as hypo-glycemic treatment for the prevention of diabetes type II, a series of 26 plant extracts were used to screen for natural inhibitors of the sodium-dependent glucose transporter SGLT1. After micro injection of cRNA of SGLT1 in Xenopus laevis oocytes, 2-electrode voltage-clamp experiments recorded the resorption of glucose in the presence of plant extracts. The ethanolic extract of Gymnema sylvestre (Retz.) showed the highest inhibition. Bioassay-guided fractionation in combination with LC-ToF-MS and 1D-/2D-NMR spectroscopy revealed 3-O-b-D-glucuronopyranosyl-21-O-2-tigloyl-22-O-2-tigloyl-gymnemagenin (1) and -21-O-2-methylbutyryl-22-O-2-tigloyl-gymnemagenin (2) as the most potent SGLT1 inhibitors (Fig. 2). Both saponines showed with 5.97 (1) and 0.17 μM (2) very low IC50-values which are comparable to the highly affine inhibitor phlorizin (0.21 μM). As the brush border membranes of SGLT1 of the intestinal epithelial cells are highly expressed, the results showed, for the first time, the potential of single saponines on the inhibition of the glucose resorption in the gastrointestinal tract.
Selected publications:
Jarolim, K.; del Favero, G.; Pahlke, G.; Dostal, V.; Zimmermann, K.; Heiss, E.; Ellmer, D.; Stark, T.D.; Hofmann, T.; Marko, D. (2017) Activation of the Nrf2-ARE pathway by the Alternaria alternata mycotoxins Altertoxin I and II. Arch. Toxicol., 91(1), 203-216. DOI 10.1007/s00204-016-1726-7.
Jarolim, K.; del Favero, G.; Ellmer, D.; Stark, T.D.; Hofmann, T.; Sulyok, M.; Humpf, H.-U.; Marko, D. (2017) Dual effectiveness of Alternaria but not Fusarium mycotoxins against human topoisomerase II and bacterial gyrase. Arch. Toxicol., 91(4), 2007-2016. DOI 10.1007/s00204-016-1855-z.
Stark, T.D.; Salger, M.; Frank, O.; Balemba, O.B.; Wakamatsu, J.; Hofmann, T. (2015) Antioxidative compounds from Garcinia buchananii stem bark. J. Nat. Prod., doi:10.1021/np5007873.
Stark, T.D.; Marxen, S.; Rütschle, A.; Lücking, G.; Frenzel, E.; Scherer, S.; Ehling-Schulz, M.; Hofmann, T. (2015) Multiparametric quantitation of the Bacillus cereus toxins Cereulide and Isocereulides A-G in foods. J. Agric. Food Chem., 63, 8307-8313.
Stark, T.D.; Marxen, S.; Rütschle, A.; Lücking, G.; Frenzel, E.; Scherer, S.; Ehling-Schulz, M.; Hofmann, T. (2015) Depsipeptide Intermediates Interrogate Proposed Biosynthesis of Cereulide, the Emetic Toxin of Bacillus cereus. Scientific Reports, 5, 10637-10651.
Marxen, S.; Stark, T.D., Frenzel, E.; Rütschle, A.; Lücking, G.; Prüstinger, G.; Pohl E.E., Scherer, S.; Ehling-Schulz, M.; Hofmann, T. (2015) Chemodiversity of cereulide, the emetic toxin of Bacillus cereus. Anal. Bioanal. Chem., 407, 2439-2453.
Balemba, O.B.; Stark, T.D.; Lösch, S.; Patterson, S.; McMillan, J. S.; Mawe, G. M.; Hofmann, T. (2014) (2R,3S,2''R,3''R)-manniflavanone, a new gastrointestinal smooth muscle L-type calcium channel inhibitor, which underlies the spasmolytic properties of Garcinia buchananii stem bark extract. J. Smooth Muscle Res., 50, 48-65.
Stark, T.; Marxen, S.; Rütschle, A.; Lücking, G.; Scherer, S.; Ehling-Schulz, M.; Hofmann, T. (2013) Mass spectrometric profiling of Bacillus cereus strains and quantitation of the emetic toxine cereulide by means of stable isotope dilution analysis and HEp-2 bioassay. Anal. Bioanal. Chem., 405, 191-201.
Stark, T.D.; Matsutomo, T.; Lösch, S.; Boakye, P.A.; Balemba, O.B.; Pasilis, S.P.; Hofmann, T. (2012) Isolation and structure elucidation of highly antioxidative 3,8’’-linked biflavanones and flavanon-C-glycosides from Garcinia buchananii bark. J. Agric. Food Chem., 60, 2053-2062.
Stark, T.; Bauer, T.; Hofmann, T.; Ehling-Schulz M. (2010) Development of a stable isotope dilution analysis (SIDA) for the quantification of the Bacillus cereus toxin cereulide in foods. J. Agric. Food Chem., 58, 1420-1428.