(Bio)organic Synthesis
Organic synthesis of reference compounds is performed (i) to unequivocally confirm the chemical structure and stereochemistry of a previously not reported natural product, (ii) to perform bioactivity studies with suitable amounts of a target molecule, and (iii) to perform accurate quantitative analyses using stable isotope labeled twin molecules of the target analytes as internal standards.
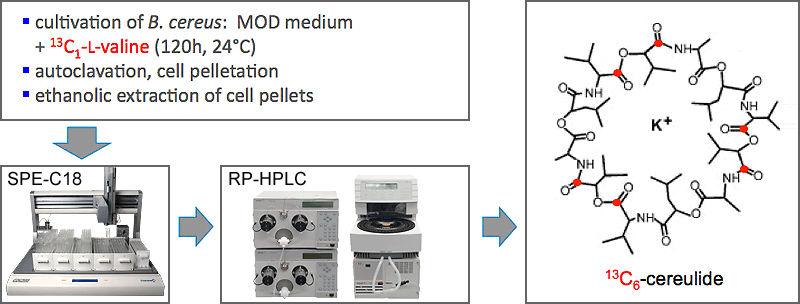
If suitable, target compounds are synthesized by using the metabolic power of microorganisms fed with suitable precursor molecules. For the first time, this biosynthetic approach successfully allowed the synthesis of a 13C-labelled twin molecule of cereulide, the emetic toxin of Bacillus cereus, and enabled the development of a stable isotope dilution analysis.